41 which of the following is not an fda required component of a food packaging label?
Nutrition Labels 101: What's Required? What's Optional? 5. Trans Fat. Trans fat is the worst of the "bad fats," which is why in 2006 the FDA began requiring it to be listed separately on nutrition labels. Trans fat, like saturated fat, increases LDL cholesterol levels (i.e., "bad" cholesterol) but also lowers HDL cholesterol levels (i.e., "good" cholesterol). So it's a bit of a ... List of ingredients and allergens on food labels - Canadian Food ... Wax coating compounds, their components and other protective edible coatings are not required to be shown on the labels of prepackaged fresh fruits or fresh vegetables as an ingredient or a component [B.01.008 (3) (a), FDR ]. Apples, turnips and cucumbers are examples of fruits and vegetables that have wax coatings.
Nutrition chap 12 quiz Flashcards | Quizlet The Nutrition Labeling and Education Act of 1990 requires which of the following? A. restaurants must provide nutrition details to consumers B. most foods be labeled using a standardized format C. all food be labeled with calorie amounts D. packaged food will include nutrient claims E. all health claims must be placed on the front of the packaging

Which of the following is not an fda required component of a food packaging label?
Homework 2 Flashcards | Quizlet the USDA Food Patterns Since 1990, food labels have been required on all fresh meat and poultry. False The net contents of a package must be reported by weight only. False There is no truly healthful way to "eat out" away from home. False Food Product Labeling Basics | Oklahoma State University Required Elements of a Food Label The Federal Food, Drug and Cosmetic Act (FD&C) requires five elements to appear on a food label: Name of the food Net quantity of contents Name and address of the manufacturer Statement of ingredients Nutrition information Chapter 5: Food Labels Flashcards | Quizlet This fact remains true whether or not the milk proclaims it. What are the eight common allergens that have to be listed on food labels? 1) milk 2) eggs 3) fish 4) shellfish 5) tree nuts (cashews, walnuts, almonds, etc.) 6) peanuts 7) wheat 8) soybeans What are the three main reasons that food labels are so important?
Which of the following is not an fda required component of a food packaging label?. Fair Packaging and Labeling Act: Regulations Under Section 4 of the ... The Fair Packaging and Labeling Act (FPLA or Act), enacted in 1967, directs the Federal Trade Commission and the Food and Drug Administration to issue regulations requiring that all "consumer commodities" be labeled to disclose net contents, identity of commodity, and name and place of business of the product's manufacturer, packer, or distributor. Packaging and Labeling - Food and Drug Administration a) Gang-printed labeling is a sheet of labeling that contains ¨ Different drug products, strengths, or net contents of same drug b) Gang-printed sheets are prohibited unless well differentiated ¨... eCFR :: 21 CFR Part 111 -- Current Good Manufacturing Practice in ... Product complaint means any communication that contains any allegation, written, electronic, or oral, expressing concern, for any reason, with the quality of a dietary supplement, that could be related to current good manufacturing practice. Examples of product complaints are: Foul odor, off taste, illness or injury, disintegration time, color variation, tablet size or size variation, under ... Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and...
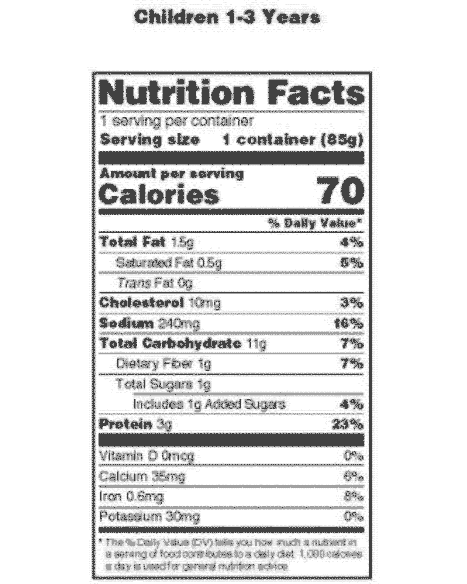
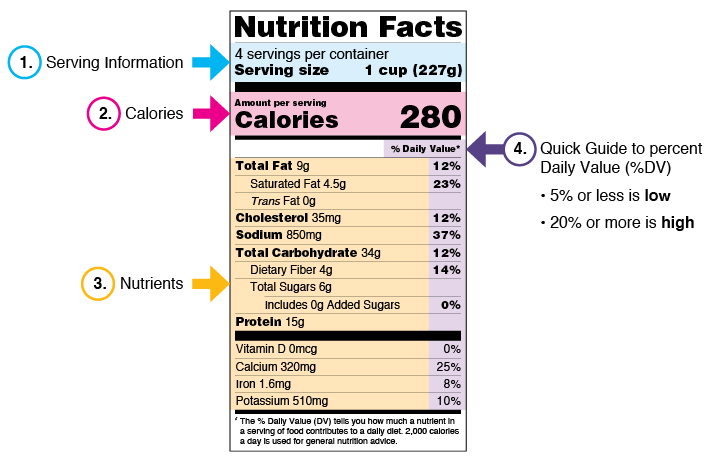
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 211.122 Materials examination and usage criteria. (a) There shall be written procedures describing in sufficient detail the receipt, identification, storage, handling, sampling, examination, and/or testing of labeling and packaging materials; such written procedures shall be followed. Labeling and packaging materials shall be ... MasteringNutrition 2d Flashcards | Quizlet which of following is an FDA required component of a food packaging label? food and drug administration (FDA) the agency that regulates the information on a food label is the... zero trans fat the FDA put seventeen companies on notice for printing misleading claims on food packaging. what type of claim did they take issue with? meat and poultry Nutrition Facts Labeling — FDA Reader The nutrition information label must include some mandatory components (i.e. calories, fat) and may include other voluntary components (vitamin A). No other declarations of nutrition information is allowed on the label, other than those listed below: Mandatory Nutrient Components Calories "Fat" or "Total Fat" Saturated Fat Trans Fat "Cholesterol" CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). CURRENT GOOD MANUFACTURING PRACTICE IN MANUFACTURING, PACKAGING, LABELING, OR HOLDING OPERATIONS FOR DIETARY SUPPLEMENTS. Subpart I - Production and Process Control System: Requirements for the Batch Production Record.
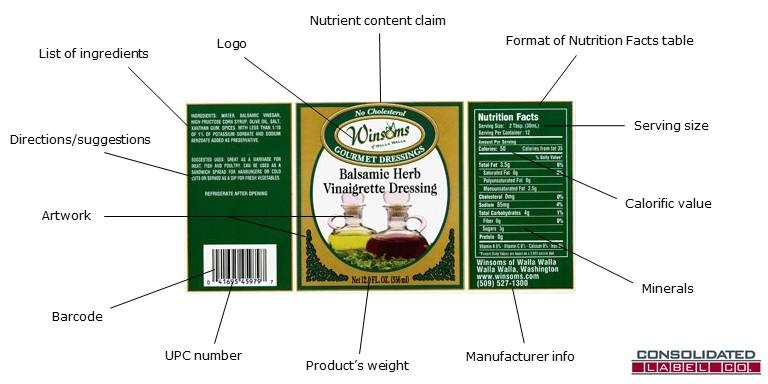
PDF Food Labeling Guide - Food and Drug Administration Office of Nutrition, Labeling, and Dietary Supplements HFS-800 Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Paint Branch Parkway College Park, MD 20740 (Tel)... Key Elements of a Food Label To Know | Food Labeling Info Although the FDA doesn't test the nutrition of every food product, you must report accurate information on your label. Below is a list of information that should be on your product's Principal Display Panel (the area most likely to be seen by consumers) in order to comply with labeling regulations: Food ingredients; Minerals; Caloric value Food Ingredients & Packaging | FDA Irradiation of Food & Packaging FDA provides regulatory and scientific information about irradiated food and packaging. Irradiation may be used to increase shelf-life and reduce harmful bacteria in... Packaging Guidelines in 21 Code of Federal Regulations Part 211 Packaging for over-the-counter drugs. Packaging for over-the-counter drugs is also specified in 21 Code of Federal Regulations Part 211, Subpart G. Per Code of Federal Regulations Part 211, Subpart G, all over-the-counter drugs should be in tamper-evident packaging. This package should have at least one barrier to entry that could indicate to ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). PART 109 -- UNAVOIDABLE CONTAMINANTS IN FOOD FOR HUMAN CONSUMPTION AND FOOD-PACKAGING MATERIAL. Sec. 109.16 Ornamental and decorative ceramicware. (a) Lead is a toxic metal that is used as a component of glazes and decorative decals on ...
The Regulation of Food Packaging | PackagingLaw.com First, FDA circulated a proposal in 1969 that it would not require a food additive regulation for substances without toxicological concern migrating to food in quantities no greater than 50 parts per billion (ppb). While this proposal was never formally adopted by FDA, it did take on a life of its own, and became known as the "Ramsey Proposal."
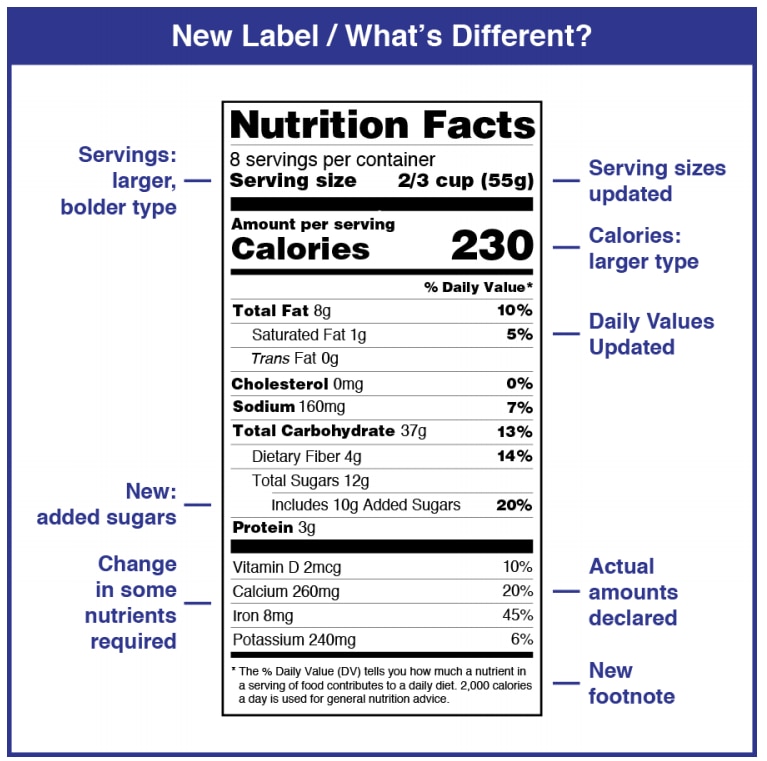
Changes to the Nutrition Facts Label | FDA - U.S. Food and Drug ... Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). CURRENT GOOD MANUFACTURING PRACTICE IN MANUFACTURING, PACKAGING, LABELING, OR HOLDING OPERATIONS FOR DIETARY SUPPLEMENTS. Subpart L - Production and Process Control System: Requirements for Packaging and Labeling Operations.
PDF SUMMARY OF 5 REQUIRED FOOD LABEL COMPONENTS Label Layout Instructions ... the PDP. Positioning and type size for each component is tightly regulated. In addition, all IP components must be placed together without intervening material, starting at the top left of the panel. PDP 1. Product Identity 21 CFR 101.3 Must include the standard food name (for a standardized food) or a descriptive name (for a non-
FDA Compliant, Food Grade and Food Safe | ISM - Industrial Spec FDA compliant means more than just safe for food contactIn general, saying a material is FDA compliant indicates that it is a food grade material. It is made of the correct type and quality of material that makes it safe for food contact. To be completely FDA compliant, the component end user must also be certain that
FDA Food Product Labeling & Packaging Requirements - ESHA The Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) mandates that packaged food items must declare, in plain language, the presence of any major food allergens (Milk, Egg, Fish, Crustacean shellfish, Tree nuts, Wheat, Peanuts, Soybeans, Sesame) on the product packaging.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 211.180 General requirements. (a) Any production, control, or distribution record that is required to be maintained in compliance with this part and is specifically associated with a batch of a drug product shall be retained for at least 1 year after the expiration date of the batch or, in the case of certain OTC drug products lacking ...
Packaging, Labeling, Transporting, Storing — Food Law Label Approval FDA does not pre-approve labels; FDA may offer suggestions if the processor inquires; FDA will enforce law after the label is put in use. No pre-approval of label is required by FDA for products under its jurisdiction. It is the responsibility of the manufacturer or importer of a food to comply with current food labeling ...
Nutrition- Chapter 2 Mastering health Flashcards | Quizlet The FDA requires the manufacturer's, packer's, or distributor's name and address to be listed on a food package. Which of the following is NOT an FDA required component of a food packaging label? A. net weight or volume of the product B. nutrient claim C. list of ingredients D. Nutrition Facts Pane Answer: B
eCFR :: 21 CFR Part 101 -- Food Labeling § 101.1 Principal display panel of package form food. The term principal display panel as it applies to food in package form and as used in this part, means the part of a label that is most likely to be displayed, presented, shown, or examined under customary conditions of display for retail sale. The principal display panel shall be large enough to accommodate all the mandatory label ...
Chapter 5: Food Labels Flashcards | Quizlet This fact remains true whether or not the milk proclaims it. What are the eight common allergens that have to be listed on food labels? 1) milk 2) eggs 3) fish 4) shellfish 5) tree nuts (cashews, walnuts, almonds, etc.) 6) peanuts 7) wheat 8) soybeans What are the three main reasons that food labels are so important?
Food Product Labeling Basics | Oklahoma State University Required Elements of a Food Label The Federal Food, Drug and Cosmetic Act (FD&C) requires five elements to appear on a food label: Name of the food Net quantity of contents Name and address of the manufacturer Statement of ingredients Nutrition information
Homework 2 Flashcards | Quizlet the USDA Food Patterns Since 1990, food labels have been required on all fresh meat and poultry. False The net contents of a package must be reported by weight only. False There is no truly healthful way to "eat out" away from home. False























![Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow](https://uploads-ssl.webflow.com/5f59aa263c234bb74025de57/5fa501736e36530857745ed8_Inner-images-8.jpg)








Post a Comment for "41 which of the following is not an fda required component of a food packaging label?"